Concise guide outlines how to write electron configuration, explains quantum numbers and shell and relationship to the periodic table.
CONTENTS
Quantum numbers and shells
Filling the shells
Relationship with the periodic table
How to write electron configuration: Notation
The electron configuration indicates the distribution of electrons in the electron shell of an atom to different energy states (orbitals).
Quantum numbers and shells
The state of each electron of the sheath is determined according to the Sommerfeld atomic model and the orbital model by four quantum numbers.
| Quantum number | character | Range of values | description | example |
|---|---|---|---|---|
| Principal quantum number | n | 1, 2, 3, … | K, L, M, … | 3 |
| Minor quantum number | l | 0, …, n-1 | s, p, d, f, … | 0, 1, 2 |
| magnetic quantum number | m | -l, …, l | 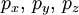 | -2, -1, 0, 1, 2 |
| Spin quantum number | s | -1/2, +1/2 | -1/2, +1/2 |
According to the Pauli principle , the state of any pair of electrons in an atom must not coincide in all four quantum numbers. This is the reason that the electrons are distributed to the various permitted states and thus to the shells and subshells.
The main quantum numbers form the shells, the secondary quantum numbers form the subshells. Each shell can be populated with a maximum of 2n² electrons according to the restrictions of l, m and s. The shells are designated in ascending order with K, L, M, N, O.
The outermost, occupied shell ( valence shell ) determines the chemical behavior and is therefore the yardstick for classification in the periodic table.
Filling the shells
With an increasing number of electrons in the elements, the possible states of their energy are occupied beginning with the lower energies. According to Hund’s rules, the orbitals of the same energy are first assigned once, then twice.
The lower shells are filled in the following order (arranged in rows):
1s (1st period) 2s 2p (2nd period) 3s 3p (3rd period) 4s 3d 4p (4th period) 5s 4d 5p (5th period) 6s 4f 5d 6p (6th period) 7s 5f 6d ... (7th period)
There are several exceptions to this order, including:
- In the case of lanthanum, an electron first occupies an orbital of the 5d subshell before 4f is filled, in the case of actinium an electron occupies 6d before 5f is filled. The electrons first occupy empty orbitals within a subshell.
- In the case of copper and chromium, an electron of the 4s orbital changes to the 3d orbital, so that the 4s orbital is only single occupied despite its lower energy level. However, the d orbitals are half (chrome) or fully (copper) occupied.
- Further exceptions are: Nb, Mo, Tc, Ru, Rh, Pd, Ag, Ir, Pt, Au, Gd and some actinides : Ac to Np and Cm.
Relation to the periodic table
In the periodic table , the occupation of the s orbital of a new shell corresponds to the jump into a new period. Within a period, the s orbitals (2 electrons – 1st and 2nd main group (exception: helium)) are occupied first and the p orbitals (6 electrons – 3rd to 8th main group) last. The subgroups correspond to the occupation of the d orbitals (10 electrons – 10 subgroups). The lanthanoids and actinides correspond to the occupation of the f orbitals (14 electrons).
How to write electron configuration: notation
The electron configuration of an atom is described by specifying the occupied subshells. The number of the shell is followed by the letter for the lower shell and superscript the number of electrons in the shell. For the second subshell (p or l = 1) of the third shell (M or n = 3) occupied by 5 electrons, the notation 3p 5 results . If there are several lower shells, the common shell is omitted. 2s 2 2p 3 becomes 2s 2 p 3.
Another abbreviated notation is obtained by putting the abbreviation for the previous noble gas in square brackets and then specifying the lower shells that are still missing for the desired element. This is used in the periodic table because of its brevity: Example: Chlorine: 1s 2 2s 2 2p 6 3s 2 3p 5 -> [Ne] 3s 2 3p 5. In addition, the clear graphical representation of the cell notation (box model) is common.