Steps and simple rules for how to find oxidation numbers used for describing oxidation-reduction (redox) reactions, their specifications and auxiliary rules.
Find more education guides, tips and advice
Oxidation numbers are formal parameters for describing oxidation-reduction (redox) reactions. They are written in Roman numerals over the element symbols. The change in oxidation numbers is the characteristic feature of redox reactions, and this is the key to how to find oxidation number.
There are simple rules for determining the oxidation number. In the case of more complex compounds or particles, the oxidation numbers of the atoms are determined using the Lewis formula, by formally performing a heterolytic bond cleavage.
Redox reactions are reactions with electron transfer between the reaction partners. The electron transfer is only immediately recognizable when ions are formed. With covalent connections only common electron pairs are formed, which are more strongly attracted by the electronegative partner. The model of oxidation numbers is used to quantitatively describe the electron transfer as well.
To this end, it is formally assumed that all substances – including the covalent compounds – are made up of so-called atomic ions. You set up the Lewis formula and mentally assign both electrons of a polar atomic bond to the more electronegative partner. This conceptual approach corresponds to a heterolytic bond cleavage.
Binding partners with the same electronegativity share the binding electrons according to a homolytic formation cleavage. Electron pairs that are not involved in the bonds (free electron pairs) remain with the associated atom.
It should be emphasized that the mental splitting of ties is only a formalism, the ties are not split in reality. However, the oxidation numbers can now be determined from the number of electrons of the formally formed atomic ions .
To do this, one compares the number of valence electrons of the neutral atom in the PSE with the number of electrons that were mentally assigned to it using the Lewis formula.
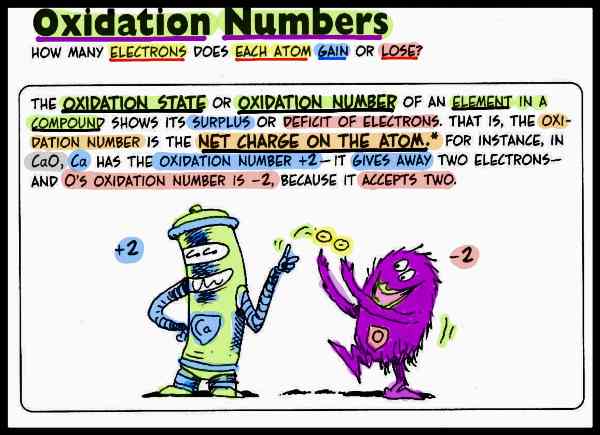
The oxidation number (Nox) of an atom in a compound is the difference between the valence electron number of the neutral atom and the number of electrons of the formally formed atomic ion. The Nox are written in Roman numerals over the element symbols. Negative Nox are given a negative sign. However, they do not correspond to real charges. The change in the Nox by one unit corresponds to the uptake or release of an electron.
Thus, the oxidation number Nox (also oxidation state, oxidation value) indicates how many elementary charges an atom has formally taken up or given off within a compound, for example in a redox reaction. It therefore corresponds to the hypothetical ionic charge of an atom in a molecule or the actual charge of single-atom ions.
Another definition reads: The oxidation number of an atom in a chemical compound is formally a measure for specifying the ratio of the electron density around this atom. A positive oxidation number indicates that the electron density has been reduced compared to its normal state, a negative one indicates that the electron density around the atom has increased.
The oxidation number is a useful formalism for chemical considerations that often has little to do with the real charge of an atom. It is quite possible that atoms in a compound are assigned a negative formal oxidation number, although they also carry a positive formal charge. The oxidation number differs from the concept of valency in covalent compounds.
How to find oxidation numbers: Specification
Oxidation numbers are written in Roman numerals over the atomic symbols in compounds (e.g. O −II ). If the element symbol is on its own, they are often written as Arabic numerals as with ions. According to IUPAC, signs are only set for negative oxidation numbers.
How to find oxidation numbers: steps
The oxidation number can be derived using the following rules:
- Atoms in the elementary state always have the oxidation number 0 (but 0 is also possible in compounds).
- In the case of monatomic ions, the oxidation number corresponds to the ion charge.
- The sum of the oxidation numbers of all atoms of a polyatomic neutral compound is equal to 0.
- The sum of the oxidation numbers of all atoms of a polyatomic ion is equal to the total charge of this ion.
- In the case of covalently formulated compounds (so-called valence line formulas, Lewis formulas), the connection is formally divided into ions. It is assumed that the electrons involved in a bond are completely taken over by the more electronegative atom.
- Most elements can occur in several oxidation states.
How to find oxidation numbers: Auxiliary rules
In practice it has proven to be helpful to formulate a few rules for determining the oxidation number:
- The fluorine atom (F) as an element with the highest electronegativity always has the oxidation number −I in compounds.
- Oxygen atoms get the oxidation number −II – except in peroxides (then: −I) and in connection with fluorine (then: + II).
- Other halogen atoms (such as chlorine, bromine, iodine) generally have the oxidation number (−I), except in connection with oxygen or a halogen that is higher in the periodic table.
- Metal atoms in compounds as ions always have a positive oxidation number.
- Alkali metals always have + I and alkaline earth metals always + II as the oxidation number.
- Hydrogen atoms get the oxidation number + I, except when hydrogen is directly connected to more “electropositive” atoms such as metals ( hydrides ) or to itself).
- In the elementary state, the oxidation number is always 0 (e.g. I 2 , C, O 2 , P 4 , S 8 ).
- In ionic compounds (salts) the sum of the oxidation numbers is identical to the ionic charge .
- In covalent connections (molecules) the binding electrons are assigned to the more electronegative binding partner. Identical binding partners each receive half of the binding electrons. The oxidation number therefore corresponds to the assigned binding electrons compared to the number of external electrons normally present.
- The highest possible oxidation number of an element corresponds to the main or subgroup number in the periodic table (PSE).
Graphic determination of oxidation numbers
Take phosphoric acid (H 3 PO 4 ) as an example :
- First the Lewis formula is recorded.
- Then the electrons are assigned to the atoms according to electronegativity
- The oxidation number can then be calculated based on the valence electrons. Example: Oxygen normally has 6 valence electrons (VI. Main group). Due to the higher electronegativity of oxygen, the binding electrons between oxygen and hydrogen (or phosphorus) can be assigned to oxygen. In the balance sheet, the oxygen receives two additional electrons in addition to the 6 available. Hence the oxidation number is −II. The phosphorus is in the main group V, thus has normally five valence electrons. Since these are all assigned to oxygen, it “lacks” five electrons and it receives the oxidation number + V.
Another example shows on the one hand how one and the same atom (here the carbon atom) has different oxidation numbers, and on the other hand how oxidation numbers change during the reaction.




